Botox FDA-approved for forehead lines
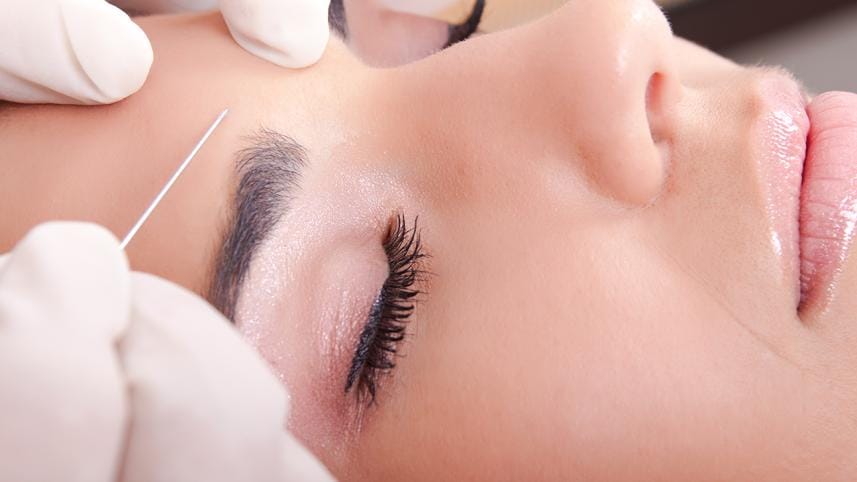
Botox has been approved by the US Food and Drug Administration for use in reducing the appearance of forehead wrinkles.
According to the pharmaceutical company Allergan, which makes the drug, the news makes the brand the only neurotoxin in the US to get approval for three facial treatment areas -- forehead lines, crow's feet lines and glabellar lines (referring to the skin between the eyebrows and above the nose).
"Allergan recognizes that forehead lines are a top area of concern for patients," said David Nicholson, Chief Research and Development Officer at Allergan, in a statement. "Our goal in pursuing a third indication for Botox Cosmetic for the temporary improvement in the appearance of moderate to severe forehead lines was based on our desire to study the patient selection, dosing and injection pattern to help provide optimal treatment outcomes."
Botox, a prescription medicine that is injected into muscles to temporarily improve the look of certain moderate to severe facial lines, first gained FDA approval back in 2002 for use on glabellar lines. A year later it was approved for use on crow's feet, and the product is now approved for use in over 75 countries. Several celebrities, from Nicole Kidman to Kim Kardashian, have admitted trying out the procedure in the past.
Clinical trials reported that Botox Cosmetic demonstrated efficacy in reducing the severity of forehead lines, when compared with placebo.



 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel.
Comments