Directorate General of Drug Administration
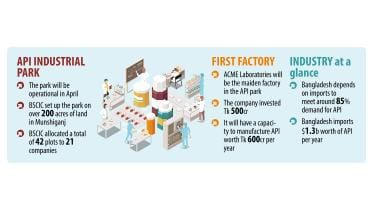
Production at API Industrial Park to begin next month
Production of active pharmaceutical ingredients (APIs) at the API Industrial Park in Gazaria of Munshiganj will begin next month, with ACME Laboratories set to commission its manufacturing unit despite the lack of gas supply.
17 March 2024, 01:08 AM
Children's death from syrup: DGDA asked to give Tk 15 lakh to each family
The High Court today directed the Directorate General of Drug Administration (DGDA) to give Tk 15 lakh as compensation to each of the families of the 104 children, who died after taking toxic paracetamol syrup in 1991 and 2009.
2 June 2022, 09:03 AM
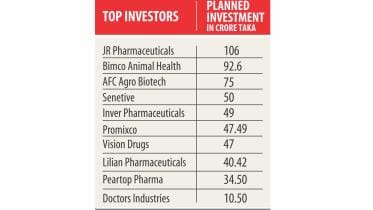
19 firms to invest Tk 650cr in pharma
The pharmaceuticals industry is gearing up to expand as 19 companies have got the go-ahead in the last one year to set up facilities at a combined investment of around Tk 654.82 crore.
27 March 2019, 18:00 PM
Mini-labs in six districts
The Directorate General of Drug Administration (DGDA) has launched six international standard mini-labs in as many districts to enhance its capacity to check sale of fake medicines.
4 January 2019, 18:00 PM
Toxic Paracetamol: HC summons health secretary Aug 23
High Court summons Health Secretary Sirajul Haque Khan to appear before it on August 23 to explain why action was not taken against two officials of the drug administration.
21 August 2017, 12:07 PM
Poor quality medicines
We are informed by the Directorate General of Drug Administration that some 153 sub-standard drugs have been detected, of which, 43 had already been marketed and awaiting registration.
27 August 2015, 18:00 PM
‘Legal action if banned drugs found in market’
The drug administration will take legal actions against the pharmaceutical companies who did not withdraw three types of drugs, banned by the government recently, from the market.
26 August 2015, 11:41 AM