Pioneering cancer drug combination approved
A pioneering pair of cancer drugs that unleash the immune system on tumours will be paid for by the NHS in England.
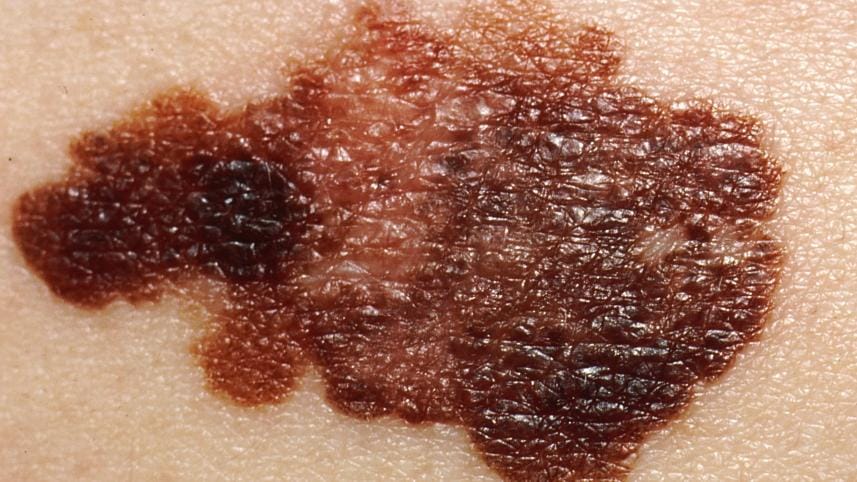
In trials, the combination therapy shrank the most aggressive and deadly type of skin cancer in 69 percent of patients.
The decision to approve the drugs is one of the fastest in NHS history and is likely to be adopted throughout the UK.
Experts said harnessing the body's own defenses was now giving "new hope" to cancer patients.
The field - known as immunotherapy - is one of the most exciting in cancer treatment.
Cancers are a corrupted version of healthy tissue and evolve ways of evading the immune system.
Ipilimumab and nivolumab stop the cancers hiding and allow the immune system to attack.
Ten years ago, patients with advanced and aggressive melanoma lived for an average of nine months.
But two years after a being given both drugs - more than half experience tumours shrinking and a fifth have no sign of cancer at all.
Prof Carole Longson, from the National Institute for Health and Care Excellence (NICE) which approved the drugs, said: "These promising new immunotherapy treatments for advanced melanoma look set to significantly extend the life of people with the condition."
The combination was licensed across Europe only last month. The speed with which it was approved by NICE means patients in England will be the first to have access to the therapy on the continent.
Peter Waite, a 63-year-old motor technician from Preston, was diagnosed with kidney cancer last year.
He started a trial on the drugs, which are now being tried in a wide range of cancers, in April 2015.
He told the BBC: "There was a 30% reduction in the size of the tumours and it has arrested any further growth.
"It's very easy to decide your life is over when you have a terminal illness, the introduction of these drugs is going to bring a lot of hope to people and I'm totally positive and looking forward to watching my grandchildren grow up."
He now has his cancer monitored every six weeks to ensure it does not start to grow again.
Nivolumab and ipilimumab both work by interrupting the chemical signals that cancers use to convince the immune system they are healthy tissue.
Nivolumab blocks the off-switch on white blood cells called PD-1. Ipilimumab blocks a similar switch called CTLA-4.
Dr Paul Nathan, from the Mount Vernon Cancer Centre in Middlesex, told the BBC News website: "Immunotherapy is genuinely exciting, it is starting to have a profound effect on many cancers and I'm in no doubt there will be patients that have long-term durable control of their disease... it really is a game-changer."
It is the dual-action that means the combination therapy works better than either alone.
However, the combination also causes inflammation in the bowels and liver as the drugs triggers the immune system to attack healthy tissues.
Prof Peter Johnson, the chief clinician at Cancer Research UK, said: "These results give new hope to melanoma patients.
"But, it's important to remember that more powerful treatment comes with an increased chance of severe side effects.
"Our research now needs to identify which patients are most likely to benefit from this combination and who is most likely to experience the side effects, so doctors can make sure we get the balance right."
Gill Nuttall, the founder of the charity Melanoma UK, added: "Once melanoma reaches an advanced stage, it is an aggressive and life-threatening disease which is difficult to treat because it has spread to other parts of the body.
"Today's decision is hugely significant for patients."



 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel.
Comments