icddr,b develops rapid diagnostic test kit for cholera
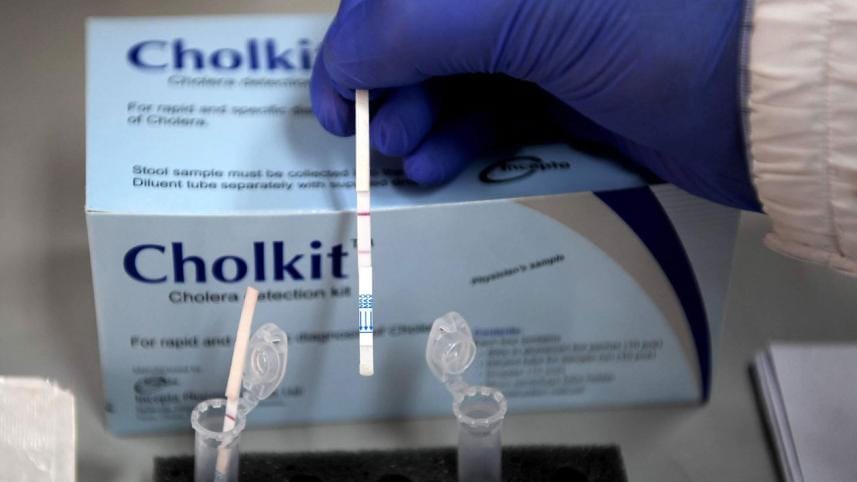
Scientists at the icddr,b, in partnership with Incepta Pharmaceuticals, have developed a locally-made low-cost dipstick device -- Cholkit -- for rapid and effective diagnosis of cholera, generating new hopes of better management the disease.
If commercially produced, the development of the dipstick will reduce the dependence on imported cholera testing kits and will open the avenue of export as well, scientist said.
The device is dipped into a tube with stool specimen and provides qualitative result (coloured band) readable by the naked eye within a maximum of 15 minutes' time.
"Presently, in addition to the laboratory culture of stool samples, imported rapid diagnostic test (RDT) kits are being used for cholera detection," said Dr Firdausi Qadri, a scientist in the Infectious Diseases Division at icddr,b, who led the development of Cholkit.
Its implication is high for the fact that globally, an estimated 1.3 billion people are at risk of cholera among which South Asians constituting the largest share. In Bangladesh, at least 66 million people are at risk of cholera, with nearly 110,000 cases reported annually, according to a statement of icddr,b today.
Scientists said following a rigorous three-year research and development process, the rapid diagnostic test (RDT) has successfully met requirements and guidelines for such tests, which are capable of detecting Vibrio cholerae from stools.
A field evaluation of Cholkit has recently been published in the scientific journal PLOS Neglected Tropical Diseases that showed the sensitivity and specificity of the dipstick is similar to the commercially available rapid diagnostic test (RDTs) when used in field settings for Vibrio cholerae from stool specimens.
A total of 7,720 stool samples were tested during the evaluation where Cholkit has shown sensitivity of 76 percent and specificity of over 90 percent while other RDTs showed around 72 percent and 86.8 percent respectively.
The gold standard for detecting cholera is laboratory confirmation by stool culture, which is sensitive to several factors, including the quality of sampling, delays in shipment, laboratory equipment, and skilled human resources. It also needs longer period of time (24 to 72 hours) and costs $6 to $8 per sample, icddr,b says.
From the public health perspective, the management of cholera outbreaks needs immediate detection as the pathogen has immense potential to spread and cause epidemics in a short period of time, it said.
"Thus, the need for simple and easy to use RDTs which are quickly interpretable, require simple storage facilities, and are reasonably priced is a clear choice," icddr,b said.
It has the potential to be used at the primary health care level for cholera surveillance, for early outbreak detection and as a tool for an initial alert for monitoring of seasonal peaks in highly endemic areas and in peripheral health care facilities, it said.
Cholkit is found to be highly efficient in detecting V. cholerae serogroup O1 and has received official licensure, it is priced around 3 US dollars, said icddr,b.
Dr Firdausi Qadri said studies have found management of cholera outbreaks depend on early detection of cholera cases.
Now that Bangladesh has a locally-made device, it would help early detection of cholera, while the country has the potentials to create new avenues for export to other endemic countries in the future, she said.
"Thus, Bangladesh will have completed locally-produced cholera preventions tools - a vaccine and a rapid diagnostic test to combat this ancient disease," she added.
The Incepta-produced Cholkit is now being used in 22 cholera sentinel surveillance sites across Bangladesh. The sites are managed jointly by icddr,b and Institute of Epidemiology Disease Control And Research.



 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel.
Comments